Snapshot
- An estimated 130 million chlamydia cases occur each year.
- Chlamydia can lead to pelvic inflammatory disease, ectopic pregnancy, and infertility, when left untreated.
- Infants born to pregnant people with chlamydia may be born early and may have eye or lung infections.
Key Challenges
- Current chlamydia prevention options include using condoms during vaginal, anal, or oral sex and abstinence. Additional prevention options, like vaccines, are urgently needed.
- Chlamydia testing is effective in detecting infections to treat and prevent further transmission. However, current diagnostics are expensive and inaccessible to many.
Chlamydia Advocacy Needs
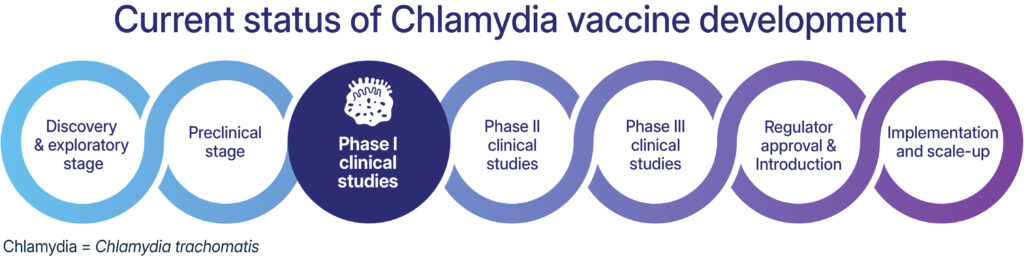
- Are vaccines available to prevent chlamydia? No, but several clinical trials are being conducted to develop a chlamydia vaccine.
- Are tests available to detect chlamydia? Yes, point of care tests are available but can be more expensive.
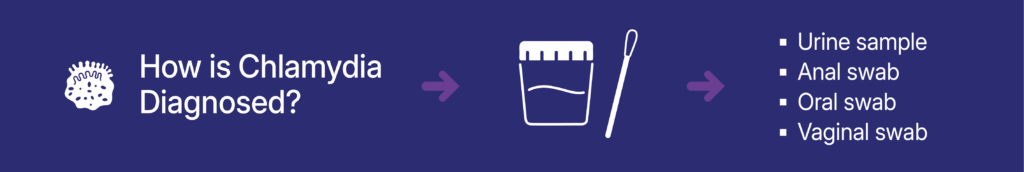
- How is chlamydia detected? Urine samples and anal, oral, and vaginal swabs can be used to detect chlamydia.
- Can chlamydia be treated? Yes, but reinfections can occur.
Vaccines
Despite decades of research, the world still lacks a chlamydia vaccine. In recent years, there have been great advancements in chlamydia vaccine development. For example, Statens Serum Institut and Imperial College London have published positive findings from phase 1 safety and immunogenicity studies of vaccine candidate CTH522, and in early 2024 the US National Institutes of Health awarded several grants for research into chlamydia vaccine development, including to the University of New Mexico and the University of Texas.
Advocacy Needs
- More funding to advance R&D for chlamydia vaccines is needed: a little more than $8 million is currently invested in this globally each year.
- Community Engagement: inclusion of communities in clinical trial design and research to explore vaccine attitudes for future implementation.
Testing
For many healthcare centers, these tests are conducted in laboratories that are often far away from where a person receives care, lengthening time to results and any needed treatment. There are tests that can be performed at the point of care, returning results within 30 minutes. These tests are generally, smaller and can fit on a table in a healthcare facility, making it easier to implement where patients receive care. Additionally, self-collection kits, where individuals collect a urine sample or vaginal swab and mail it to a laboratory for testing, provide an additional option for people to engage in STI testing in and outside of the clinic setting.
Syndromic management, diagnosing chlamydia based on symptoms, is often used when testing is unavailable. However, this approach can lead to the overtreatment and undertreatment of chlamydia infections because most people do not experience symptoms and symptoms they do experience can be confused with other infections like gonorrhea.
How Chlamydia is Detected
Chlamydia is diagnosed using molecular tests, which can detect genetic material from the bacteria that causes chlamydia (C. trachomatis, or CT) from a urine sample or vaginal, anal or throat swab. Research has shown that vaginal, anal, and throat swabs are more effective at detecting infections compared to a urine sample. Studies have shown that urine-only testing could miss 75% of infections.
Advocacy Needs
- Affordable and accessible point-of-care tests are needed for implementation in a broad range of health care settings to test and treat chlamydia in a single healthcare visit.
- Increased awareness on how to detect and test for chlamydia including by using urine samples and vaginal, anal, and oral swabs.
Treatment
Chlamydia can be cured with commonly available antibiotics, usually doxycycline or azithromycin.