Snapshot
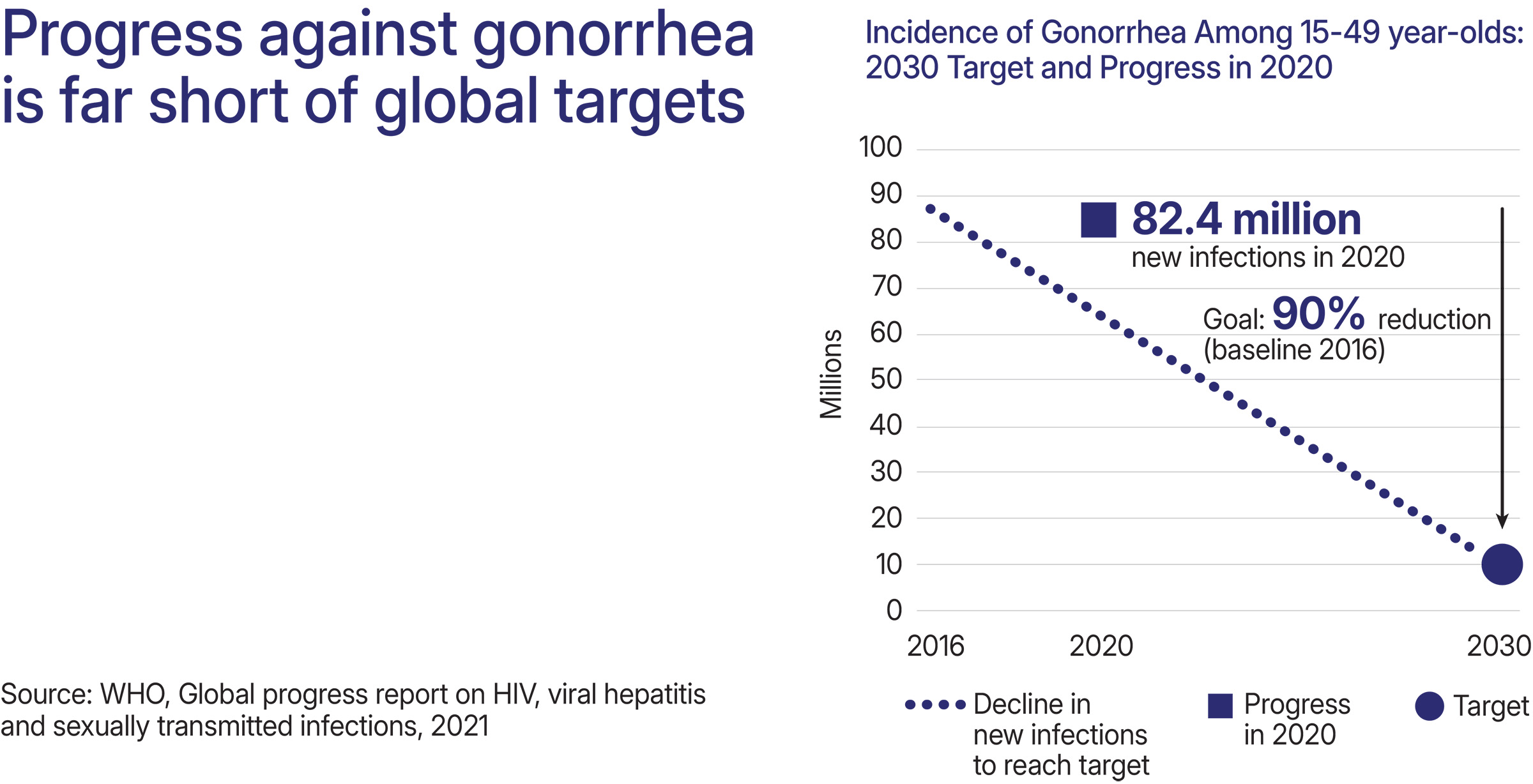
- Approximately 82 million gonorrhea cases occur each year.
- Most infections occur in the African and Western Pacific regions where prevention and treatment options are limited.
- Antimicrobial resistance threatens to make gonorrhea untreatable.
- If not treated, gonorrhea can lead to serious consequences including pelvic inflammatory disease, ectopic pregnancy, and infertility.
- Gonorrhea infections significantly increase susceptibility to HIV.
Key Challenges
- The world is running out of gonorrhea treatment options.
- Diagnosing gonorrhea takes too long and costs too much.
- There’s no vaccine to prevent gonorrhea and current prevention options are limited.
Gonorrhea Advocacy Needs
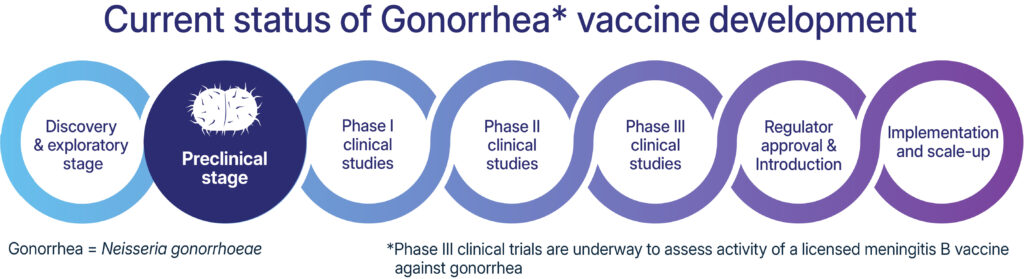
- Are gonorrhea vaccines available? No, but several clinical trials are being conducted to develop a gonorrhea vaccine. A meningococcal group B vaccine has also been shown to reduce gonorrhea infections.
- Are tests available to detect gonorrhea? Yes, point of care tests are available but can be more expensive than laboratory tests.
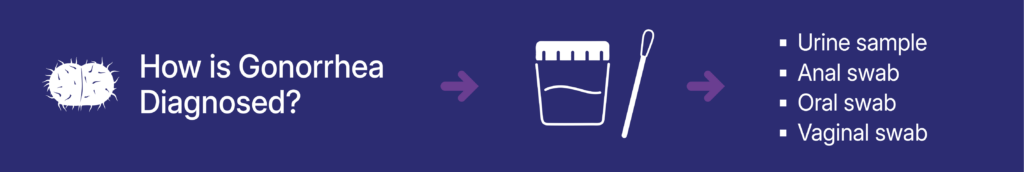
- How is gonorrhea detected? Urine samples and vaginal, anal, and oral swabs can be used to detect gonorrhea.
- Can gonorrhea be treated? Yes, but has acquired resistance to nearly every class of antibiotic.
Overview
Gonorrhea is caused by the bacteria, Neisseria gonorrhoeae. Infections can be cured with antibiotics, but over the decades it has acquired resistance to nearly every medicine used to treat it. Additionally, gonorrhea infections are typically asymptomatic, especially among women, highlighting the importance of early detection to limit the spread of the infection.
Vaccines
Researchers believe that a gonorrhea vaccine is possible, particularly because epidemiological evidence shows that some vaccines against meningitis B, which is caused by a pathogen closely related to the bacteria causing gonorrhea, may reduce new gonorrhea infections. Clinical trials are underway to study the effectiveness of meningitis B vaccines against gonorrhea. Several other, different types of gonorrhea vaccine candidates are in preclinical development.
Diagnosis
Gonorrhea testing includes both culture and nonculture methods. Historically, culture tests were considered the gold standard to detect gonorrhea, but NAAT tests, a nonculture method, are now considered the gold standard after demonstrating high sensitivity and specificity. Additionally, NAATs can often conduct tens to hundreds of tests at a time.
Many newer diagnostics have combined chlamydia and gonorrhea tests to detect both at once. Point-of-care (POC) and near-POC tests that can be used in community and clinical settings are becoming more available. These tests can help to limit the time between test and treatment, limiting the spread of infections.
Treatment
Gonorrhea can be cured with antibiotics, but over the decades it has acquired resistance to nearly every medicine used to treat it. Currently only one class of antibiotics (cephalosporins) remains widely effective against the infection, and even within this class, treatment failures have been reported in Australia, France, Japan, Slovenia, Sweden, and the United Kingdom. Gonorrhea is evolving antimicrobial resistance across lower-income countries as well, but this is less well documented due to a lack of adequate surveillance data.
Although WHO calls antimicrobial resistance a top global health threat, the world faces an inadequate pipeline of new antibiotics and inequitable access to existing medicines. While research continues into a number of potential new treatment options, more sophisticated diagnostics that can assess the susceptibility of an infection to a range of current and older medications could help preserve the effectiveness of the treatments we have now.
Advocacy Needs
- Better surveillance data is needed to assess the effectiveness of today’s treatment options in lower-income countries.
- Diagnostics that can detect resistance are needed to ensure proper treatment of gonorrhea infections.
- Alternative treatments that have shown efficacy, such as gentamicin, must be made available and affordable in lower income countries.
- Advocates should continue to press for investment in antibiotic R&D, including further research into promising gonorrhea treatment candidates such as zoliflodacin, solithromycin, and gepotidacin.