STI Roadmap
Collaboration, coordination, communication and advocacy are essential to catalyze vaccine development action.
The World Health Organization, the US National Institutes of Health, and global partners have published a comprehensive roadmap for development of new STI vaccines. The roadmap identifies three key workstreams:
Make the case for STI vaccine investment
- What public health need would the vaccine address?
- How valuable would the vaccine be?
Expedite research and development
- Will it take to develop an effective vaccine?
Optimize global benefits and access
- What should the vaccine look like to optimize its benefits?
- Who will get it and how will it be used?
- What can be done in parallel with product development to ensure speedy, targeted rollout of vaccines so they are accessible to those who would benefit the most?
Together, these workstreams describe an end-to-end approach that promises to guide investment, accelerate development of new vaccines and ensure faster, more targeted rollout of vaccines to those who could benefit the most.
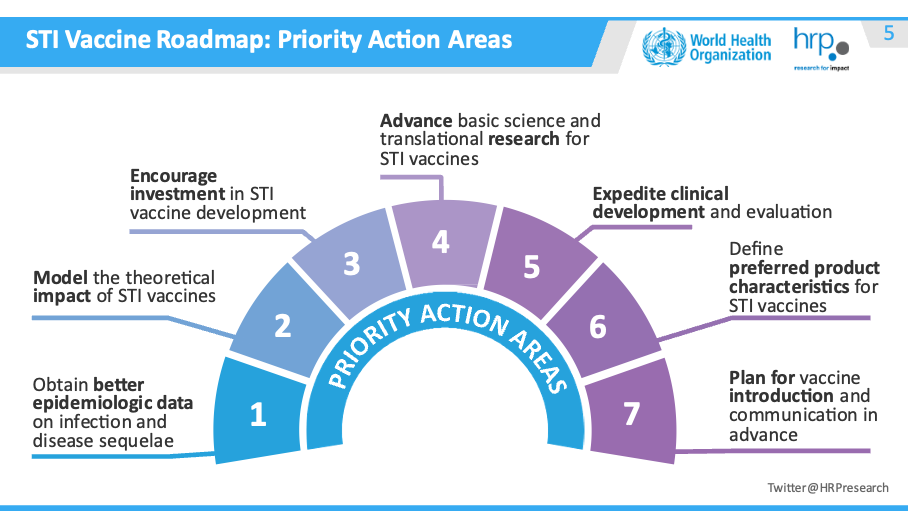
Progress along the roadmap differs by potential STI vaccine.
- Obtain better epidemiological data
- Model the theoretical impact of STI vaccines – underway for gonorrhea and HSV
- Encourage investment in STI vaccine development – NIH awards granted for syphilis, gonorrhea and chlamydia vaccine research
- Advance basic science and translational research
- Expedite clinical development and evaluation
- Define preferred product characteristics – completed for HSV and gonorrhea
- Plan for vaccine introduction and communication
Collaboration, coordination, communication and advocacy are essential to build on this current momentum and further catalyze vaccine development action.
Learn More:
- WHO Fact Sheet on STIs
- Gottlieb SL, Johnston C. Future prospects for new vaccines against sexually transmitted infections. Curr Opin Infect Dis. 2017 Feb;30(1):77-86. doi: 10.1097/QCO.0000000000000343.
- Gottlieb SL, Deal CD, Giersing B, et al. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016 Jun 3;34(26):2939-2947. doi: 10.1016/j.vaccine.2016.03.111
- Broutet N, Fruth U, Deal C et al. (2014). Vaccines against sexually transmitted infections: The way forward. Vaccine. 32. 1630–1637. 10.1016/j.vaccine.2014.01.053.