Resources
Resource List
Sexually Transmitted Infections: ‘Self-testing’ versus ‘self-collection’: the critical role of consistent language in the field of STI diagnostics

Integrating HIV, Viral Hepatitis and Sexually Transmitted Infections with Primary Health Care – Learning from countries

Updated recommendations for the treatment of Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema pallidum (syphilis) and new recommendations on syphilis testing and partner services

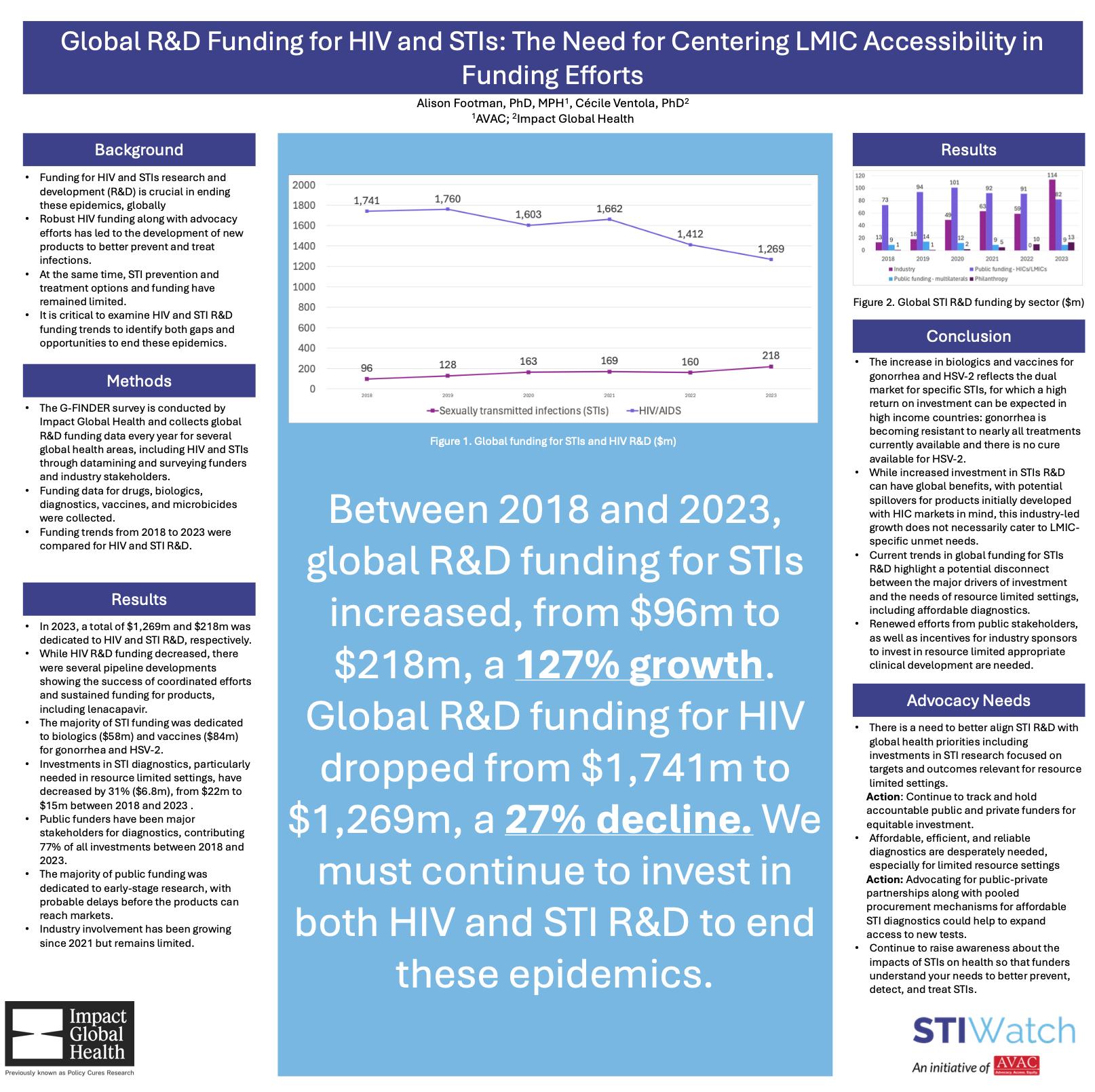
The STIWatch resource database offers a range of material on the status of STI vaccine and diagnostics research, development and availability.
STIWatch’s Clinical Trials Dashboard
The STI Clinical Trials Dashboard provides information about trials focused on vaccines, diagnostics, and the use of doxycycline post-exposure prophylaxis (DoxyPEP) to detect, treat, and prevent chlamydia, gonorrhea, hepatitis B, herpes simplex virus (HSV), human papillomavirus, syphilis and trichomoniasis infections.
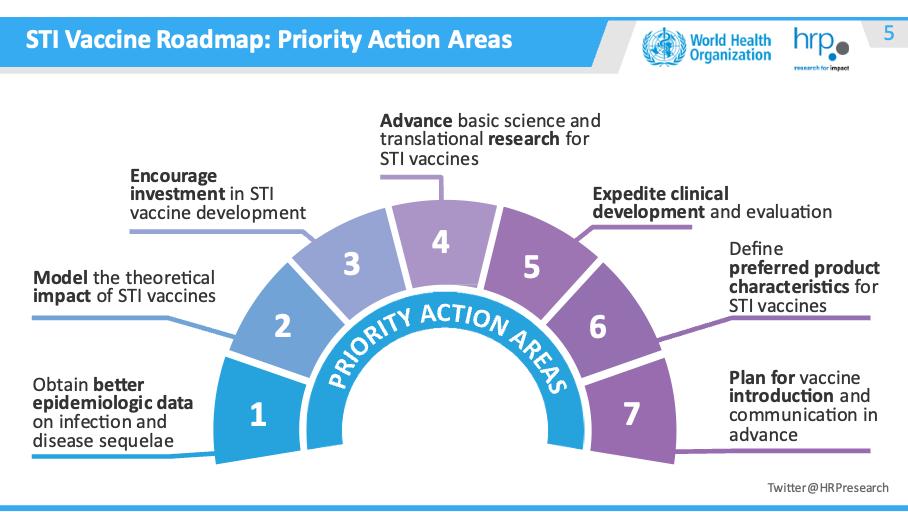
The STI Roadmap
Collaboration, coordination, communication and advocacy are essential to catalyze vaccine development action. The World Health Organization, the US National Institutes of Health, and global partners have published a comprehensive roadmap for development of new STI vaccines.
-
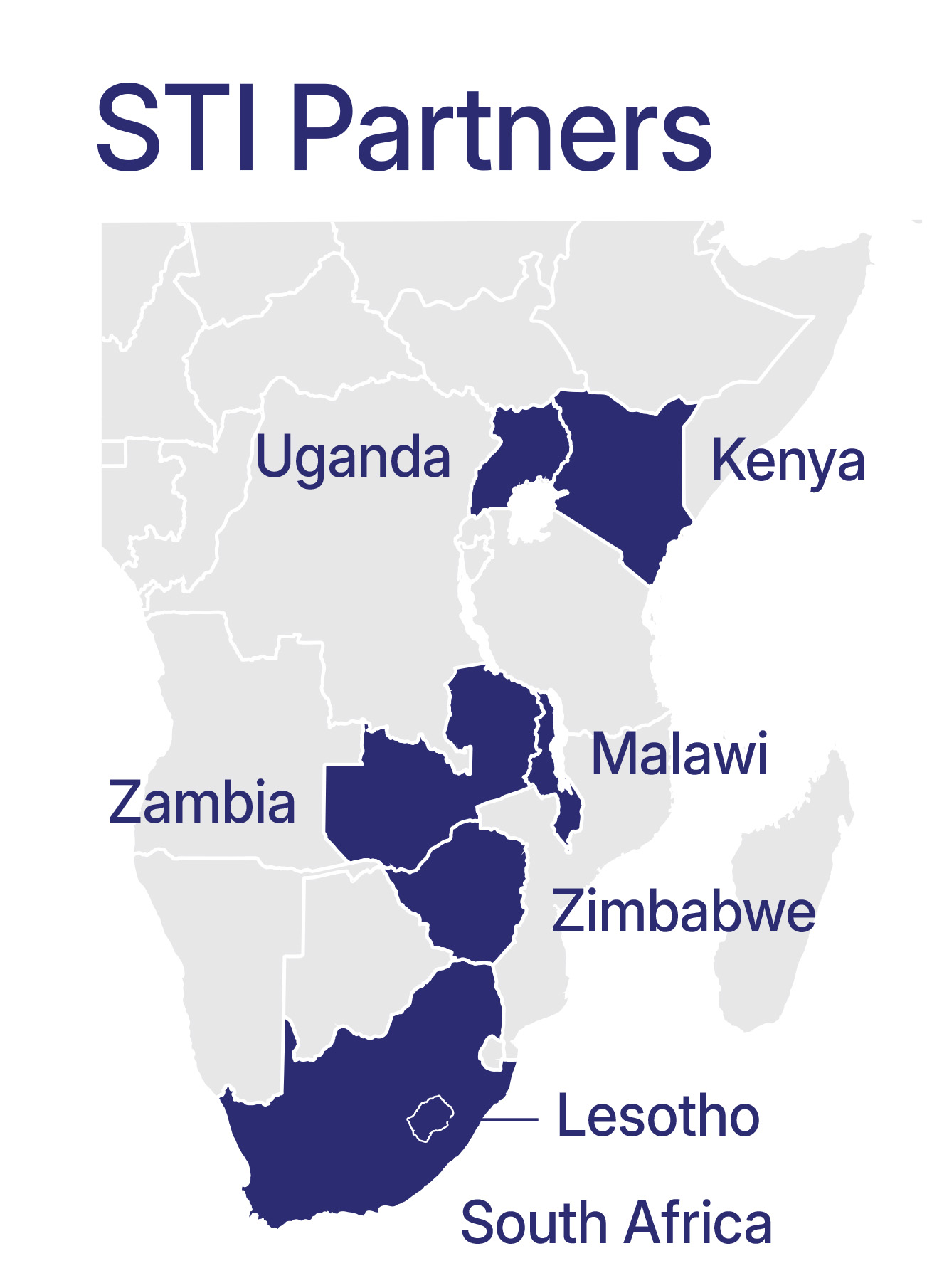
STI Advocacy in East and Southern Africa
In 2023, AVAC and partners set out to assess the STI prevention landscape across East and Southern Africa. These analyses informed a strategic advocacy agenda.